Since the creation of Abiogen Pharma, the strategic choice has been to enhance and develop the know how gained through years of manufacturing and the skills acquired in pharmaceutical development.
The Abiogen Manufacturing aim is to manage both the manufacturing and development of the projects and products of Abiogen Pharma, and the development of manufacturing for external clients.
In fact, Abiogen Pharma performs CMO and CDMO activities, supporting pharmaceutical companies as a strategic partner throughout the entire lifecycle of a drug, offering services such as: development of a drug’s formulation and technical Dossier, analytical testing, scale-up, and commercial production.
Quality assurance
Compliance to the GCP for clinical studies and the GMP for production, is constantly monitored by periodic internal inspections to the studies and the production processes. Maintenance, upgrading and continuous growth of know how is made possible by regular customer audits, representing a rare opportunity for analysis and comparison with the greatest representatives of the quality and manufacturing sector. The results of these audits through the respective services of Quality Assurance are further evidence that Abiogen Pharma’s Pharmaceutical Factory offers an adequate and consistent sphere of quality assurance.
Main chemical analysis and physical-chemical techniques
- Tin Layer Chromatography
- High Performance Liquid Chromatography (HPLC)
- Ionic Chromatography
- Gas-Chromatography
- Gas-Chromatography Head Space Tecnic
- UV-VIS Spectroscopy
- Infrared Spectroscopy (FTIR)
- Atomic Absorpion Spectrometry
- NIR Spectroscopy
- TOC Determination
- Visible and Subvisible Particolate Contamination
- Acid-base titrations
- Complexometric titrations
- Redox titrations
- Potentiometry
- Conductometry
- Polarimetry & Optical Rotation
- Microwave Mineralization
- Steam Distillation
- Physical Characterization (melting point, refractive index, density, viscosity)
- Detector Massa
- Sterility test
- Bioburden
- LAL test
- Like Heparin activity determination
- Biological assay of actives
- Loss on drying
- Disaggregation
- Dissolution
- Friability
- Tablets Hardness, Thickness, Diameter
- Disaggregation time of suppository
- Hardness of Ampoules
- Process Validation
- Technology Transfer from Development to Pilot Production
- Technology Transfer of commercial products Demonstration lots
- Analytical Transfers
- Process Validation
Quality assurance
- Product release
- Process validation
- Cleaning validation
- Deviations & CAPA
- Compliants & Recall
- Environmental & utilities monitoring
- Supplier audits
- Self Inspection
- Documentation control
- Analytical tranfer
- On-going stability studies
- Chemical analysis
- Microbiology analysis
- Sterility testing
- Computer systems validation
Integrated pharmaceutical development services
The knowledge and expertise acquired over many years of activity supporting research in the development of products from Abiogen’s product list, makes Pharmaceutical Development one of the strengths of the Business Manufacturing area.
The department consists of an area equipped for production and a quality control unit for Investigation Medicinal Products (IMPs) which operate according to the EU-GMP Good Manufacturing Practices Annex 13.
The production unit has an area for the production of IMPs for clinical phases III-IV (pilot scale).
Analytical laboratories, well equipped with the latest state-of-art machinery, assure the timely release of IMPs. The areas have been GMP accredited for Good Manufacturing Practices EU-GMP annex 13 since 2005.
- Drug substance characterisation
- Investigation on solid state properties of API
- pH / solubility profiles
- Forced degradation studies
- Excipient compatibility studies
- Compatibility Studies between API and container
- Blend characterization
- Formulation Development for early studies
- Prototype formulations for clinical trials
- Product Reformulation for Innovation & Product Life Cycle management
- Preliminary process identification
- Manufacture of pivotal stability batches
- Process scale-up (from lab. to pilot scale and from pilot to industrial scale)
- Process optimization for commercial scale manufacture
- Manufacturing and release testing of phase III and IV drug product
- Dedicated secondary packaging operations areas
- Dedicated storage area for raw materials and finished IMPs
- Tech Transfer from development R&D to pilot plant
- Tech Site Transfer of commercial products
- Demonstration batches
- Analytical Transfer
- Process Validation
- Development and Validation of analytical methods
- Dissolution and drug-release profiling
- Specifications development
- Stress testing
- Photostability testing (according to ICH)
- Formal stability (all storage condition according to ICH)
- On-going stability
- In-use stability
- MPD (Investigational Medicinal Product Dossier)
- PSF (Product Specification File, GMP Annex 13)
- CTD Quality (Common Technical Document module 3.2 / module 3) for Product Registration
- Type IA, Type IB and Type II Variations (CE 1234/2008 regulation)
- Small volume parenterals (ampoules, aseptic and terminally sterilized process)
- Blending and Granulation (High shear granulator / drier, Top spray fluid bed granulation, Fluid bed driers)
- Compression (single punch and high-speed rotary tablet presses)
- Tablet-coating (film and sugar coats)
- Capsule filling (Coni-snap® Vcaps™, DBcaps®)
- Liquid and Semi-solid (solution, suspensions, emulsion, creams, gels, lotions and ointments)
- Comparator-blinding services, including encapsulation and coating
- Placebo development for use in blinded studies
- Primary packaging in blisters, bottles and tubes
- Secondary packaging for open-label or double-blind studies
- Liquid Chromatography: HPLC system (UV, diode array, refractive index and MS detectors), HPLC-IC system, UPLC system (UV, diode array)
- Gas-Chromatography (GC & HS-GC)
- Thin-layer Chromatography (TLC)
- Absorption spectrophotometry, infrared (FT-IR)
- Absorption spectrophotometry, ultraviolet and visible (UV-VIS)
- Dissolution (automatic system)
- Disintegration test (with automatic systems)
- Potentiometric titration
- Osmolality
- Optical rotation
Contract development and manufacturing
The activities developed and the successes obtained in the last decade confirm the vision of Abiogen Pharma towards a range of services aimed at creating a real value to the projects undertaken and building a synergistic partnership with its customers.
The offer is developed through the provision of high-quality standards, significant and certified technical-scientific competence and the technological capacity available in the various strategic areas of Manufacturing, Pharmaceutical Development, Purchasing, Quality Unit, Legal Affairs, Logistic and Customer Care and a network of suppliers specialized in quality.
The projects are evaluated in their complexity and carefully analyzed according to the state of the art regulatory requirements and the value is sought for each project phase by ensuring competitive prices aligned to the European market.
Contract Manufacturing, Pharmaceutical Development services and Clinical Supply are the sectors of excellence of Abiogen Pharma’s service contracts.
Plant
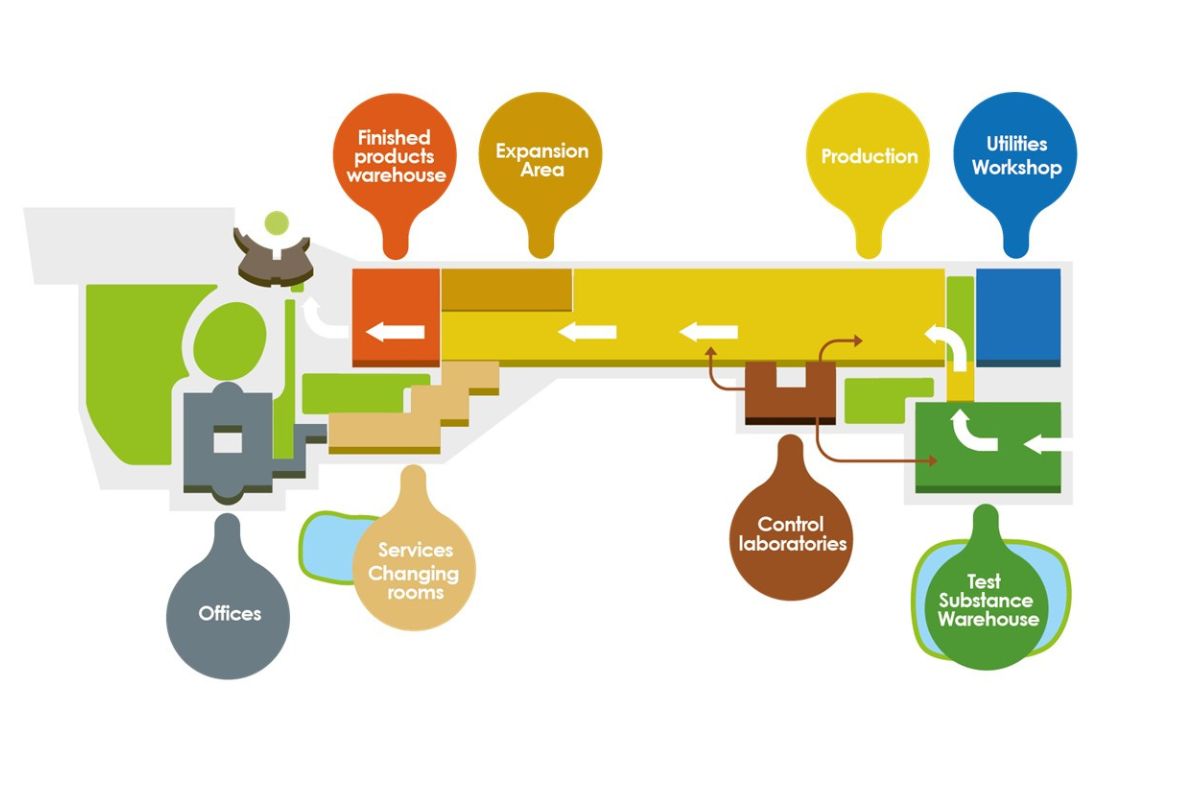
The site located on the outskirts of Pisa in Ospedaletto is spread along an area of 120.000 m2 and a covered area of 26.000 m2.
The premises were designed to align Abiogen Pharma Manufacturing to the state of the art in European pharmaceutical production and pharmaceutical development.
The flows of materials and personnel meet the strict requirements of the G.M.P (Good Manufacturing Practices) in Europe.
The layout of the site is considered to be at today’s cutting edge of new pharmaceutical plant construction. It was designed according to the concept of dedicated units per single formulation, each equipped with one or more independent HVAC systems and dedicated entrances for the personnel and materials, respecting the most stringent requirements set by the European GMP (Good Manufacturing Practice).
Two artificial lakes, built to avoid the use of tap water, secure the cooling of the industrial plants and irrigation of the green areas. Extreme attention was paid to structure rationalization with particular care of the production flows.
The entire production activity is continuously monitored through an Emerson computerized system that can detect, and, if necessary, restore in real-time all key parameters of the production equipment through digital sensors and optic transmissions.
Industrial areas of the manufacturing division
| Total plant surface | 120.000 mq. |
| Roofed surface | 26.000 mq. |
| Raw materials warehouse | 3.400 mq. |
| Finished product warehouse | 3.000 mq. |
| Pharmaceutical manufacturing | 10.000 mq. |
| Pharmaceutical development | 1.080 mq. |
| Quality control laboratories | 1.080 mq. |
| Services and Utilities | 2.000 mq. |
| Offices | 5.400 mq. |
This production area covers a surface of about 1.800 mq.
Production capacities:
350/400 tons/ year.
Orals solids process:
- High shear granulation followed by: static drying or fluid bed drying
- Fluid bed granulation
- Traditional mixing
- Direct mixing
- Tableting
- Film coating
- Hard capsules filling
Manufacturing equipment:
- Fluid-bed processor: Glatt WSG 300 1100 lt
- High shear mixer granulator: Gral 300 GEA 300 lt
- 2 IMA Cyclope Tumbler
- High Shear Mixer PMA 1200 GEA Niro Pharma System
- Tabletting: Korsch XL 400, Kikosui, Ronchi SA, Ronchi GA
- Coating: Film coating machines IMA/GS ( 2 da 300 lt e 1 da 150 lt)
- Hard capsule filling machine: Zanasi 40
- IMA Venus Component whashig machine
Packaging equipment oral solid
Production capacities:
30 million/year pieces
Type of products:
- Tablets
- Hard Capsules
- Softgel capsules
Packaging equipment:
- Line Blister 1: Marchesini Integra equipped for serialization/aggregation and tamper evidence
- Line Blister 2: Marchesini Integra equipped for serialization and tamper evidence
- Line Blister 3: IMA C90 equipped for serialization and tamper evidence
- Line Blister 4: Marchesini Integra equipped for serialization/aggregation and tamper evidence
type of blisters:
- Alu/alu
- PVC/alu
- PVC/PVDC/alu
This production area covers a surface of about 1105 mq.
Production capacities:
- 35 million/year ampoules (from 1ml to 10ml)
- The filling area is run in a class “A” environment.
Equipment:
- BOSCH filling line
- FEDEGARI autoclaves
- CEA particles inspection machines mod. A35 e mod. A35LD
- CMP LT01 Leak Test
Packaging:
- PARTENA NMX and HV CAM equipped for serialization/aggregation and tamper evidence
Type of packaging:
- PVC containers with ALU cover possibility
This production area covers a surface of about 200 mq.
Production capacities:
- 8 million/year semi solid forms: cream, Gel, Ointment, Paste
Equipment:
- Turbomixer OLSA MACEF PH1000
Packaging:
- aluminium and plastic TUBE FILLING UNIPACK 100 and PMX
This production area covers a surface of about 800 mq.
Production capacities:
45 million/year oral liquid forms: syrups, drops, lotions
Equipment:
- 2000 and 5000 and 1000 litres stainless steel vessels
- N ° 4 Marchesini high-speed influxing lines, 3 of which are suitable for multipacks and all equipped for serialization/aggregation and tamper evidence
- N°1 Comas containers packaging line equipped for serialization and tamper evidence
Type of containers:
- Packaging Glass & plastic containers
This production area covers a surface of about 800 mq.
Production capacities:
- 15 million/year pieces
Equipment:
- Num.2 Filling machine IMA Adapta
- Num.2 Sealing machine IMA Hermetica
Production capacities:
800.000/year pieces
Equipment:
- 4 tracks stick pack machine Omag.
Production equipment for clinical studies
Equipment
Twin-Pack Blistering machine O.M.A.R.
Technology
PVC/ALU – PVC-PVDC/ALU
Capability / Typical range
7 cycles/minute
Equipment
aminar Air Flow (dedicated for API of clinical phase III-IV)
Technology
Sampling and Dispensing
Equipment
Multi Processor MP1 (laboratory fluid bed dryer) GEA Niro Pharma System
Technology
Mixing, Drying, agglomeration, top spray coating
Capability / Typical range
6 Kg – 8 Kg (16 litres capacity)
Equipment
PharmaConnect® (Through-the-Wall) GEA Niro Pharma System
Equipment
Lab Mini Blender Module
Technology
Mixing
Capability / Typical range
Bin 3 litres 450 g -1,2 Kg – Bin 5 litres 750 g -2,0 Kg – Bin 15 litres 2,25 Kg -6 Kg
Equipment
High Shear Mixer
Technology
Granulation
Capability / Typical range
Develop. scale (3 litres Bowl size) 720 g -1.35 Kg – Pilot scale (30 litres Bowl size) 7.2 Kg – 13.5 Kg
Equipment
Thermotron T 7510 (Static Essiccator)
Technology
Drying
Equipment
IMILL-F 5’’ Calibrating mill IMA
Technology
Calibration of wet or dry products
Capability / Typical range
Square holes (6-8-10 mm) Mainly used for wet granulation Round holes (0.35-6 mm) Mainly used for dry granulation
Equipment
Ronchi EA8 (High-speed rotary tablet presses)
Technology
Tabletting
Capability / Typical range
4,800-24,000 tablets/hour
Equipment
Kramer E80 (De-duster)
Equipment
GS HTM/25 Tablet-coating equipment
Technology
Tablet coating
Capability / Typical range
25 litres
Equipment
Peristaltic pump Watson&Marlow, mod. 520Di
Technology
Liquid and Semi-solid (creams, gels, lotions and ointments)
Equipment
Sotax automatic tester AT 7 smart
Technology
Dissolution
Capability
2 systems






